At Sychem, we are experts in all things Decontamination.
Supplying Infection Control consumables to countless SSD units across the UK, Sychem recognise the dire importance of the Decontamination cycle taking place as it should.
What is Decontamination?
Decontamination is a series of processes designed to remove or destroy all forms of contamination. It is a critical step in device reprocessing, as instruments or devices cannot be sterilised until they are thoroughly clean.
During decontamination, soiled instruments are sorted, inspected, and disassembled if needed. Initially, instruments are manually cleaned before any capital equipment is used in the process.
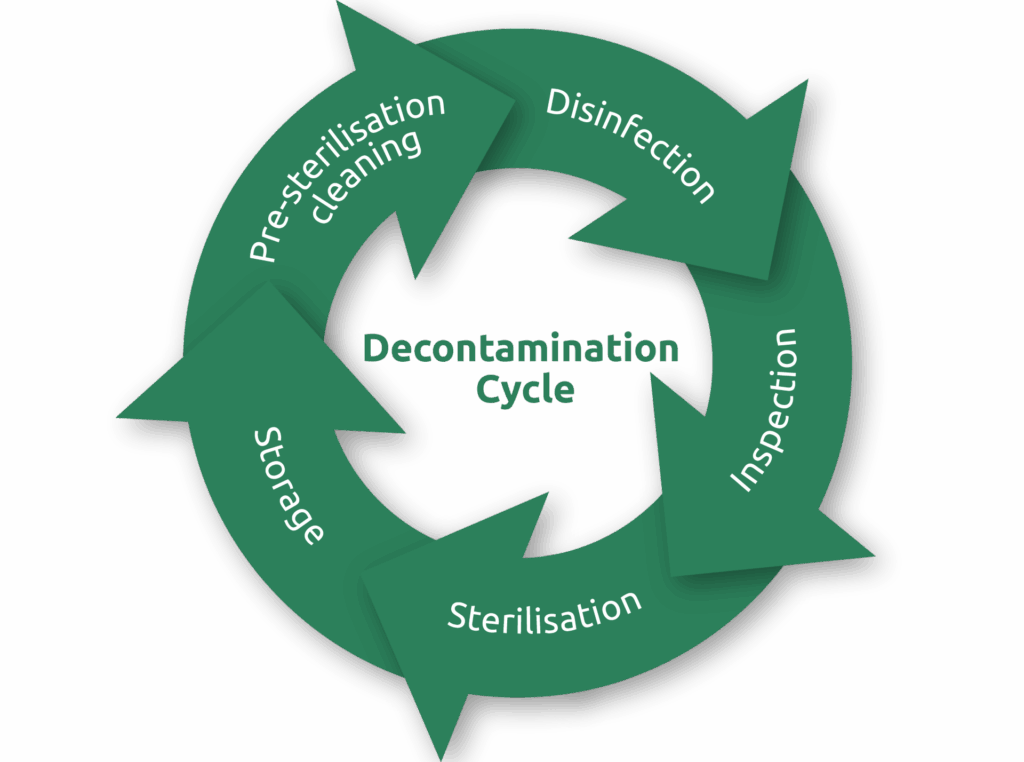
The Decontamination Cycle
The decontamination cycle consists of multiple stages, ensuring that the reprocessing of surgical instruments is carried out correctly. Each stage contributes critically to preparing instruments for safe reuse, beginning with the removal of visible soil and ending with their proper sterilised storage.
By following the steps in order, the process supports consistent cleanliness, safety and reliability throughout.
Some of the critical stages of Decontamination include:
- Pre-sterilisation cleaning
- Disinfection
- Inspection
- Sterilisation
- Storage
The Stages Explained
Pre-sterilisation cleaning and disinfection
Pre-sterilisation cleaning is the initial stage of decontamination; it is fundamental to remove all bioburden. There are three methods:
By hand
Thick rubber gloves must be worn, and the instruments should be immersed in detergent in a deep sink to prevent splashes. This manual cleaning requires either a two-bay or a three-bay sink.
- Sink 1: Instruments are immersed in an enzymatic solution to begin breaking down soils
- Sink 2: Instruments are immersed in a detergent solution and manually brushed
- Sink 3: Instruments are thoroughly rinsed with clean, treated water
A long-handled brush is recommended to clean the visible debris from the instruments. Once finished, the brush should be cleaned, autoclaved and stored dry. Brushes for this purpose should be replaced weekly.
Ultrasonic Cleaners
Ultrasonic cleaners use acoustic cavitation, forming air bubbles that implode on an instrument’s surface. These air bubbles can reach small crevices and hard-to-reach areas on a device. Ultrasonic cleaners are commonly used to clean devices that may be sensitive to damage and are too delicate for a traditional washer/disinfector.
Washer/ Disinfectors
This is the most effective of the three methods because it includes the disinfection phase. This ensures the instruments are safe for handling and inspection.

Inspection
After cleaning, all the instruments should be inspected for cleanliness and checked for damage before sterilisation. Once checked, the instruments are wrapped, ready for sterilisation.
Sterilisation
Once the instrument pack has been prepped for sterilisation, it is ready to be sterilised using one of many methods of sterilisation.
Steam sterilisation is the most widely used technique because it is effective for items that can withstand both heat and moisture. There are several types of steam sterilisation cycles, including gravity, pre-vacuum, and SFPP (Steam Flush Pressure Pulse). The total cycle time can change depending on the type of cycle used, the load’s weight and density, and factors like exposure and drying time.
At the completion of the cycle, the technician reviews the steriliser printout to confirm that all sterilisation parameters were met. Biological and chemical indicators are also used to monitor the process, helping verify that the load was exposed to the correct conditions to achieve sterility.
Storage
All sterilised instruments should be stored in a clean, dry and covered environment.
FAQs
We have compiled a list of frequently asked questions regarding the Decontamination Cycle to provide additional guidance and support for understanding each stage of the process.
Is there a difference between sterilisation and decontamination?
Decontamination reduces dirt, debris, and most microorganisms, making a device safe for use, but it does not eliminate all microbes.
Sterilisation goes further by destroying all viable organisms, including spores, ensuring the item is completely free of microbial contamination.
What are the methods of pre-sterilisation cleaning?
The three methods are:
• By hand
• Ultrasonic cleaners
• Washer/disinfectors
Why is the decontamination cycle important?
It ensures consistent cleanliness, safety, and reliability by preparing instruments for safe reuse.
What methods are used for pre-sterilisation cleaning?
The three methods are manual cleaning by hand, ultrasonic cleaners, and washer/disinfectors.
What affects the total time of a steam sterilisation cycle?
Cycle type, load weight and density, exposure time, and drying time can all affect the total cycle duration.
How is successful sterilisation confirmed?
A technician reviews the steriliser printout and uses biological and chemical indicators to verify correct sterilisation conditions.
How should sterilised instruments be stored?
Sterilised instruments should be stored in a clean, dry, and covered environment.