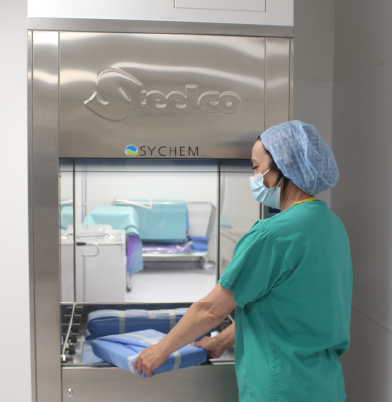
Cytostatic Negative Pressure Isolators
Designed for GMP Class A applications, the isolator’s special air guidance and glove intervention handling ensure complete separation of the operator and process. An intuitive touchscreen interface allows for seamless operation, monitoring, and control of all processes.
Home / Pharmaceutical Isolators / Cytostatic Negative Pressure Isolators

Click to see full view

Click to see full view

Click to see full view









Hover to zoom
Cytostatic Negative Pressure Isolators




Cytostatics are highly hazardous substances, predominantly classified as CMR, meaning they possess carcinogenic, mutagenic, or reprotoxic characteristics. The cytostatic isolator provides effective protection for operators while maintaining product integrity.
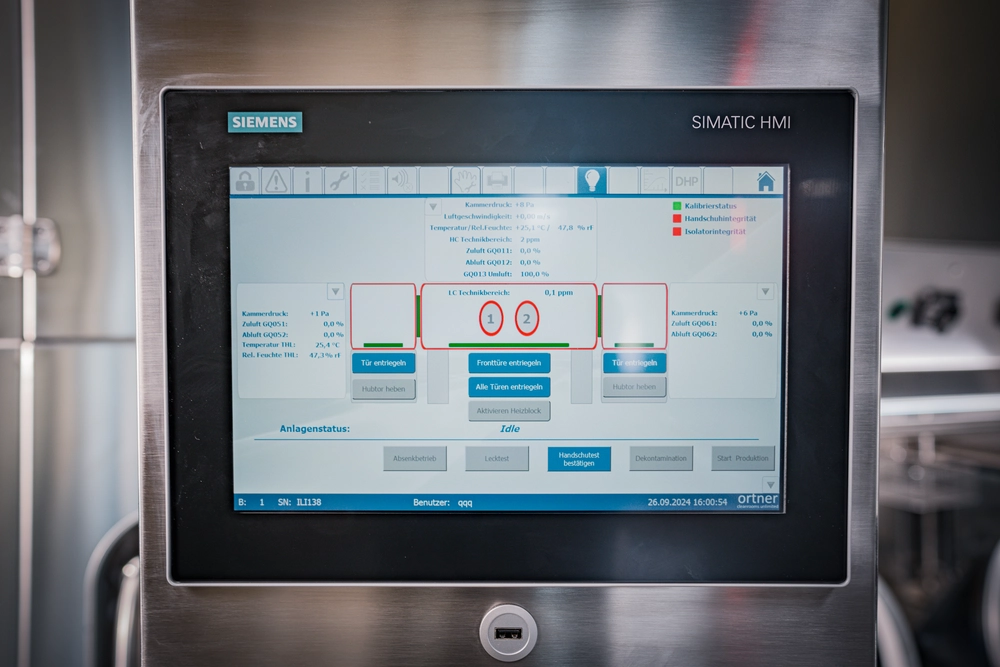
Continuous Particle Monitoring
Particle monitoring is a critical parameter in GMP monitoring and is used to monitor air purity. Ortner isolators are equipped with a fully integrated, user-friendly online particle measurement system for 0.5 μm and 5.0 μm particle sizes. The monitoring is active during production operation, with particle concentrations measured continuously throughout the entire production process.
Configurations
Specifications | Values |
|---|---|
| Model | Isolator 2-Port and Isolator 4-Port |
| Dimensions | Isolator 2-Port: 1360 x 700 x 630 (inner dimension) & 2680 x 2500 x 1000 (overall dimension) | Isolator 4-Port: 1880 x 700 x 630 (inner dimension) & 3200 x 2500 x 1000 (overall dimension) |
| Power Supply | 3/N/PE ~400 V/230 V 50 Hz; 16A/6.8 kW |
| Compressed Air | DIN ISO 8573-1 [2:2:2]; min. 6 bar |
| Supply/Exhaust Air | 260 – 600 m³/h (HVAC integration possible) |
| Monitoring | Integrated system for airflow velocity & pressure; temperature and humidity tracking |
| Continuous Particle Monitoring | Real-time detection and logging of 0.5 µm & 5.0 µm particles |
| H₂O₂ Bio-Decontamination | Fully automated sterilisation for each airlock & entire system |
| Compressed Air Process | Ensures dry operation to eliminate condensation risk during decontamination |
| Additional Options | Glove testing with port detection; online particle monitoring and temperature/humidity tracking | Integrated H₂O₂ gas generator and fully automatic material lock decontamination | Height-adjustable design; USB/RS232/230V sockets and remote maintenance access | CCTV; intercom; audit trail; OPC UA system integration and GMP/ICH9 qualification. |

Customisbale Options
- Automated glove testing & logging
- Integrated temperature & humidity monitoring
- Fully adjustable isolator height
- Intercom system with surveillance camera
- Additional airlocks with bio-decontamination
- Additional RTP ports & electrical connections
- Remote maintenance access & audit trail
- Shelf system or wire basket mounting
Downloads

Pharmaceutical The Complete Solution
Download
Case Studies
Featured Posts
Thank you for requesting a quote. A member of our team will be in touch shortly.
Thank you for requesting a quote. A member of our team will be in touch shortly.