Sychem are experts in Infection Control, and we supply a range of Consumables to various industries, to ensure the best possible levels of Infection Control are achieved.
We offer two Infection Control processes, Protein Residue Testing and ATP testing. Despite both being used to measure Infection Control levels, they are sizeable differences between the two.
What is an ATP test?
An ATP test is a Hygiene monitoring test.
Create specifically to serve the Food and Beverage Industry; an ATP test is used to monitor microbiological contaminations after the initial disinfection of surfaces and machines has taken place.
The test then found its way into the Healthcare sector after several outbreaks of Healthcare-acquired infections (HAI’s). Hospitals then began incorporating a similar method of control inside the Central Sterile Service Department (CSSD).
-

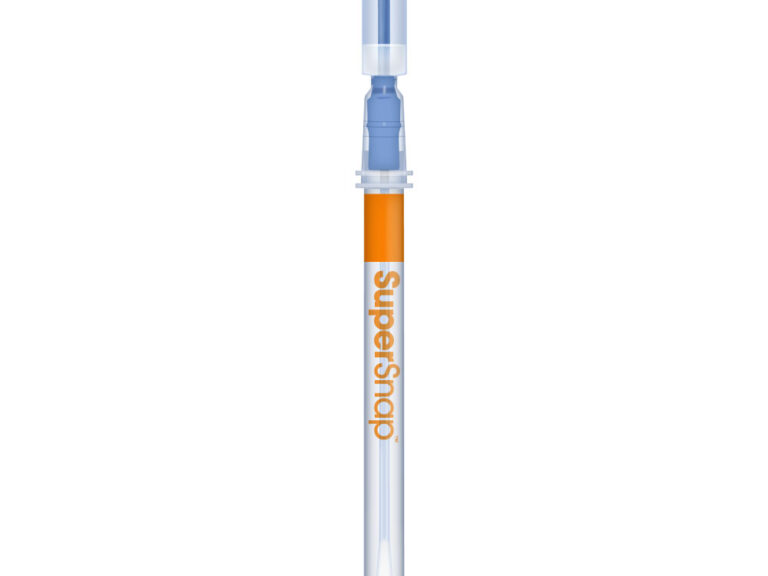
Hygiena SuperSnap™ (100 Box)
The most sensitive ATP surface test in Hygiena’s portfolio
£185.00 Add to basket -

Hygiena SystemSURE Plus
World’s best selling ATP sanitation monitoring system
£1,195.00 Add to basket -

Hygiena AquaSnap™ Total (100 Box)
AquaSnap™ Total measures both free ATP and microbial ATP in solutions
£182.00 Add to basket
Sure Trend- ATP Hygiene Monitoring
The enlistment of ATP in the Healthcare sector was a mistake. The main objective of a washing/cleaning process in hospitals is to eliminate blood and tissue remains, thus guaranteeing that subsequent disinfection and sterilisation processes will be successful.
One of the main challenges that Washer and Detergent manufacturers face is the complete removal of proteins from reprocessed instruments. Therefore, regarding testing for cleaning effectiveness, Protein Residue Testing is the best choice. The same is true for the Sterilisation process; to ensure the complete elimination of microorganisms, a Biological Indicator with bacterial spores is necessary to monitor it. In this sense, blood and tissue residues consist of both cells and proteins.
The most resistant proteins coagulate and adhere to the instruments, making the washing process difficult to successfully complete. In addition, it begs the question of the necessity of including ATP monitoring, if the instruments will be exposed to a sterilisation process to remove any living cells that remain regardless. This leaves ATP restricted to surface disinfection control in the food industry, rather than inside the CSSD.
ATP Hygiene monitoring does not represent contamination itself. Therefore, microbial or organic contamination will always be an indirect measurement when measured by ATP. On the other hand, however, Protein Residue tests directly measure organic/microbial contamination.
ATP does not present a challenge to the washing procedure, as the molecule is very easy to hydrolyse. However, hospital washing/cleaning parameters guarantee that any ATP molecule will be destroyed during the process (temperature, detergents or disinfectants, etc). Furthermore, if there is any chance that ATP molecules remain after the washing cycle, free ATP will inevitably hydrolyse within a few minutes. Therefore, it is highly unlikely that an ATP test will give a positive result after a hospital washing cycle.
Protein Residue Testing vs ATP Hygiene Monitoring
Composed entirely of a protein material that causes transmissible spongiform encephalopathies, such as variant Creutzfeldt-Jakob disease. Remarkably, proteins are one of the main components of viruses, while prions are infectious proteins themselves.
The UK Department of Health have set limits on micrograms ( g) of protein to consider an instrument as “clean” in The Health Technical Memorandum 01-01 (HTM 01-01). Therefore, it is not enough with the simple detection of proteins, but also its quantification by electronic systems of high sensitivity and temporary analysis of the evaluated samples is important.
Chemdye® PRO1 MICRO represents an easy option to monitor the cleaning of reusable medical devices in the context of medical, dental practices and industry from the detection and quantification of surface proteins, allergens and reducing agents. The system consists of a swab of special characteristics and two reactive solutions contained in the same device. PRO1 MICRO offers a quantitative colourimetric result that can be quantified with high sensitivity using any of the Bionova® Auto-readers IC10/20FRLCD and MiniPro.
To detect microorganisms by means of ATP, the available systems are only similar to PRO1MICRO in their structure. Still, the biochemical reactions are very distant because they were developed for different purposes. ATP, the energy source of microorganisms, triggers a light reaction that can be measured in a Luminometer. This system does not recognise proteins of any nature. In conclusion, proteins do fulfil all the requirements to be the marker molecule for cleaning effectiveness monitoring, making our Chemdye® PRO1 MICRO system the most reliable cleaning monitoring system in the market.
To find out more about our range of Protein Residue Testing and ATP Monitoring Systems, get in touch with one of our experts.
Visit the Terragene webpage to find out more about their full range of Infection Control consumables, or visit the Hygiena webpage to discover their extensive portfolio of ATP Hygiene Monitoring systems.